How Can PSMA Provide a Tailored Approach in Advanced Prostate Cancer?
Neal Shore, MD, FACS
Dr. Shore discusses the emerging role of prostate-specific membrane antigen (PSMA) as a diagnostic, prognostic, and potential therapeutic target, enabling a tailored approach to treating advanced prostate cancer. The overexpression of PSMA in >80% of patients with prostate cancer makes it an attractive target and a key phenotypic biomarker in advanced prostate cancer.
PSMA is a transmembrane protein that is anchored in the cell membrane of prostate cancer epithelial cells.1 PSMA has been used as a phenotypic biomarker with positron emission tomography (PET) imaging approaches.2
PSMA can be overexpressed in metastatic prostate cancer relative to normal tissue and is present in >80% of men with prostate cancer.3-8
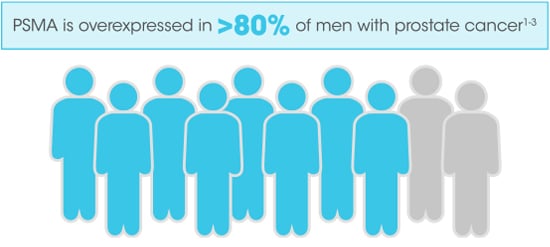
PSMA has been shown to have potential utility at multiple time points within the prostate cancer care spectrum.6,9-12

PSMA and Diagnosis
PSMA PET/computed tomography (CT) has been used to diagnose and stage prostate cancer. For men with high-risk localized disease, PET/CT had 27% (95% CI, 23-31) better accuracy than conventional imaging (92% [88-95] vs 65% [60-69]; P<0.0001) for identifying pelvic nodal or distant metastatic disease.9*
CT, computed tomography; nmCRPC, nonmetastatic castration-resistant prostate cancer; PET, positron emission tomography; PSMA, prostate-specific membrane antigen.
*The ProPSMA study was a prospective, multicenter, randomized, controlled trial of men with high-risk apparently localized prostate cancer. A total of 302 men were randomly assigned to receive either CT and bone scan (conventional imaging) or PSMA PET/CT. First-line imaging was done within 21 days of randomization. The primary outcome was accuracy of first-line imaging for identifying either pelvic nodal or distant metastatic disease defined by the receiver operating curve using a predefined reference standard including histopathology, imaging, and biochemistry at 6-month follow-up.9
PSMA and Prognosis
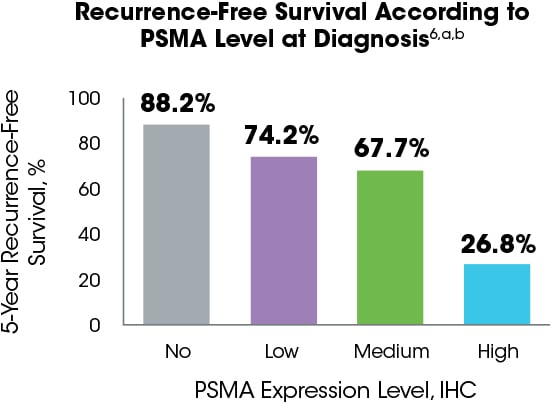
PSMA expression level in tumors has been negatively correlated with survival outcomes.6
The 5-year recurrence-free survival rates are 88.2%, 74.2%, 67.7%, and 26.8% for patients exhibiting no, low, medium, or high PSMA expression on preoperative biopsy, respectively.6
IHC, immunohistochemistry; PSMA, prostate-specific membrane antigen.
aPSMA expression was assessed in a retrospective study by immunohistochemistry in 294 preoperative biopsies, 621 primary tumor foci from 242 radical prostatectomies, 43 locally advanced or recurrent tumors obtained from transurethral prostate resection, 34 lymph node metastases, 78 distant metastases, and 52 benign prostatic samples from patients who underwent surgery. PSMA expression was categorized as no expression (score of 0), low expression (1), medium expression (2), or high expression (3). Expression was correlated with recurrence-free survival as the primary end point measure.6
bDisease recurrence was defined as biochemical recurrence (prostate-specific antigen increase above the postoperative nadir following radical prostatectomy) and used as end point for survival analysis.6
Because of their utility in prognostics, PSMA PET/CT results may be used to guide management decisions in clinical practice.11
PSMA and Clinical Management
The results of a retrospective, real-world, single-institution study showed that PSMA PET/CT after conventional imaging may lead to management changes in up to 60% of patients.11,12
PSMA PET/CT May Help Guide Clinical Management With a Tailored Approacha
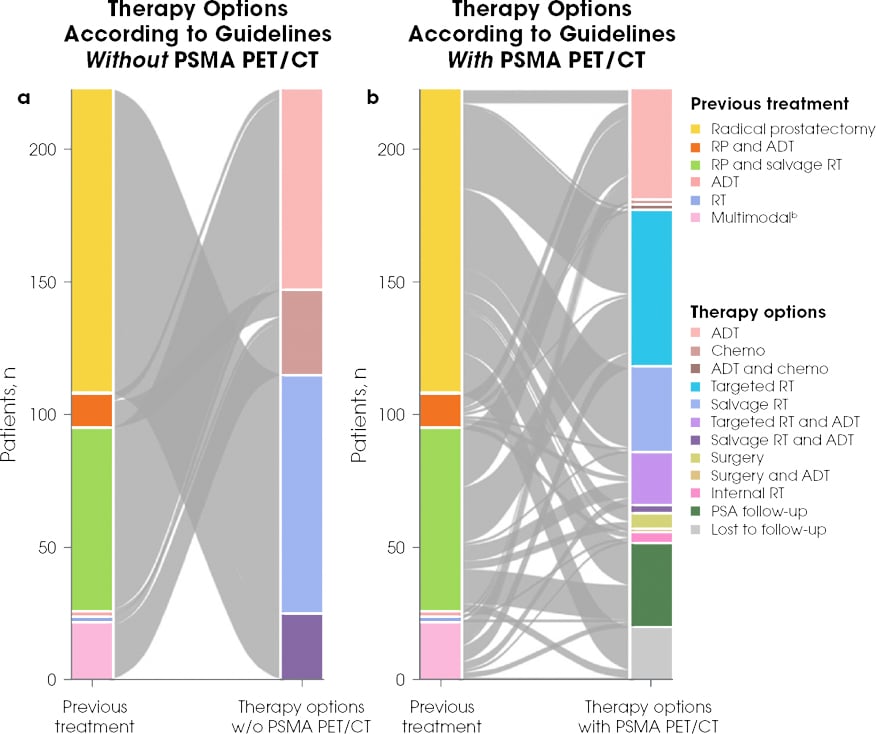
Reproduced by permission from Springer Nature: Eur J Nucl Med Mol Imaging. Müller J et al. Copyright 2019.
ADT, androgen deprivation therapy; CT, computed tomography; PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; RP, radical prostatectomy; RT, radiotherapy.
aThe aim of the retrospective study from Switzerland was to assess the effect of PSMA PET/CT on management and outcome in all patients imaged during the first year after its introduction into clinical routine. The rate of detection of recurrence was based on clinical reports. In the 203 patients with follow-up 6 months after PSMA PET/CT, the therapies effectively implemented as well as follow-up PSA levels were evaluated, with a PSA value <0.2 ng/mL representing a complete response and a decrease in PSA value of at least 50% from baseline representing a partial response.11
bMultimodal included surgery, salvage RT, ADT, and/or chemotherapy combined.11
Furthermore, based on preclinical data, PSMA has been shown to affect several key oncogenic pathways* and is being evaluated as a relevant therapeutic target.13-18

Many of these outcomes are driven by the role of PSMA in the PI3K/Akt pathway.13
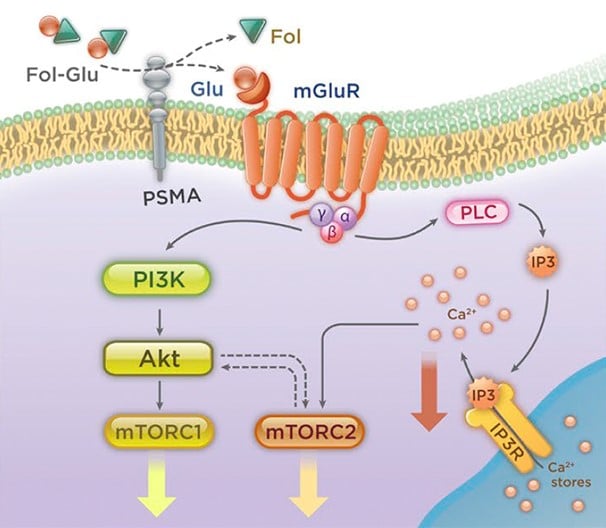
Reproduced with permission of Rockefeller University Press, from: Prostate-specific membrane antigen cleavage of vitamin 89 stimulates oncogenic signaling through metabotropic glutamate receptors. Kaittanis C et al. J Exp Med. 2018;215(1):159-175; permission conveyed through Copyright Clearance Center, Inc.
*In in vitro and animal studies.
PSMA is a diagnostic and potential therapeutic target, enabling a phenotypic precision medicine approach to treating advanced prostate cancer in the following ways6,9,11,19,20:
- Detection of a clinically relevant biomarker using a noninvasive diagnostic tool21,22
- Optimization of patient selection to help inform management decisions9,11,12,23
- Utilization of phenotypic precision medicine with the goal of improving patient outcomes11,19